Acid-Base Solutions
|
|
|
Back to HTML5 Version |
Topics
- Acids
- Bases
- Solutions
- Equilibrium
Description
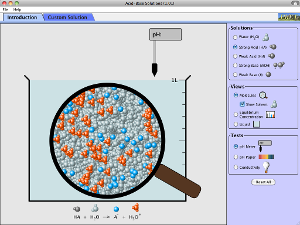
How do strong and weak acids differ? Use lab tools on your computer to find out! Dip the paper or the probe into solution to measure the pH, or put in the electrodes to measure the conductivity. Then see how concentration and strength affect pH. Can a weak acid solution have the same pH as a strong acid solution?
Sample Learning Goals
- Given acids or bases at the same concentration, demonstrate understanding of acid and base strength by: 1.Relating the strength of an acid or base to the extent to which it dissociates in water 2.Identifying all of the molecules and ions that are present in a given acid or base solution. 3.Comparing the relative concentrations of molecules and ions in weak versus strong acid (or base) solutions. 4.Describing the similarities and differences between strong acids and weak acids or strong bases and weak bases.
- Demonstrate understanding of solution concentration by: 1.Describing the similarities and differences between concentrated and dilute solutions. 2.Comparing the concentrations of all molecules and ions in concentrated versus dilute solutions of a particular acid or base.
- Use both the strength of the acid or base and the concentration of its solution in order to: 1.Describe in words and pictures (graphs or molecular drawings) what it means if you have a: Concentrated solution of a weak acid (or base) or Concentrated solution of a strong acid (or base) or other combinations. 2.Investigate different combinations of strength/concentrations that result in same pH values.
- Describe how common tools (pH meter, conductivity, pH paper) help identify whether a solution is an acid or base and strong or weak and concentrated or dilute.
Version 1.03
Keywords
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type | Subject |
|---|---|---|---|---|---|---|
| Using PhET in High School Chemistry- all my activities in pdf |
|
|
Trish Loeblein | HS UG-Intro |
Lab HW Demo |
Chemistry |
| Concept Questions for Chemistry using PhET |
|
|
Trish Loeblein | UG-Intro HS MS |
MC | Chemistry |
| Acid Concentration and Strength Investigation |
|
|
Julia Chamberlain, Susan Hendrickson | HS UG-Intro |
HW Guided |
Chemistry |
| Acid Base Solutions - Concentration and Strength |
|
|
Trish Loeblein | HS UG-Intro |
CQs Lab HW |
Chemistry |
| Strong and Weak Acids |
|
|
Kelly Lancaster, Laurie Langdon | UG-Intro HS |
Lab HW CQs |
Chemistry |
| Acid-Base Macro Particulate Symbolic |
|
Ted Clark | HS UG-Intro |
Other HW |
Chemistry | |
| PhET Sims Aligned to the Chemistry Curriculum |
|
Julia Chamberlain | UG-Intro HS |
Other | Chemistry | |
| Alignment of PhET sims with NGSS |
|
Trish Loeblein | HS | Other | Earth Science Chemistry Biology Physics |
|
| How do PhET simulations fit in my middle school program? |
|
Sarah Borenstein | MS | Other | Physics Earth Science Chemistry Biology |
|
| MS and HS TEK to Sim Alignment | Elyse Zimmer | MS HS |
Other | Biology Physics Chemistry |
||
| NGSS Simulation Alignment/Correlation | Matthew Huffine | UG-Intro K-5 MS HS |
Other Demo |
Other | ||
| Acid-Base Discovery (ICP/Physical Science Level) | S. Mitchell | HS | Lab | Chemistry | ||
| acidity PH and its influance on amino acids residual and on proteins | Nurit Hechel | HS | Guided | Biology | ||
| pH ASAM BASA | dwi sulistyorini | MS | Guided | Chemistry | ||
| Acidi e Basi | Laura Bianca Condorelli | HS | Lab Guided HW |
Chemistry | ||
| 산-염기 용액 SIM 사용지침서 | 이화국(Wha Kuk Lee) | HS UG-Intro |
Lab Demo |
Biology Chemistry Physics |
||
| Acid_Base_Investigation_PT | Luís Gaspar | HS | Lab Guided |
Chemistry |
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|