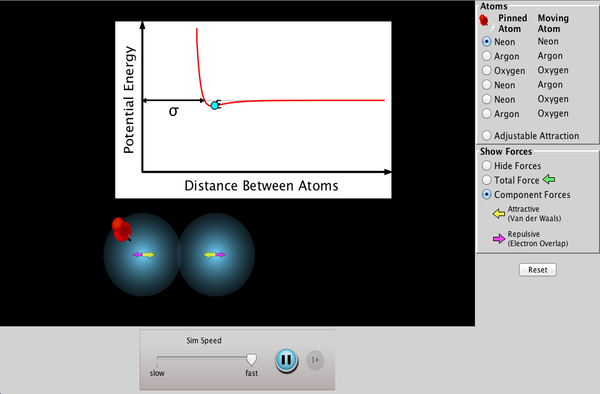
Atomic Interactions
|
|
|
Back to HTML5 Version |
Topics
- Interaction Potential
- Atomic Bonding
- Van der Waals Force
- Lennard-Jones Potential
- Pauli Repulsion
- Atomic Interactions
Description
Explore the interactions between various combinations of two atoms. Observe the the total force acting on the atoms or the individual attractive and repulsive forces. Customize the attraction to see how changing the atomic diameter and interaction strength affects the interaction.
Sample Learning Goals
- Explain how attractive and repulsive forces govern the interaction between atoms.
- Describe the effect of potential well depth on atomic interactions.
- Contrast the potential well and behavior of a bonded pair of atoms with unbonded pairs.
- Describe bonds as dynamic and bond lengths as average distances.
Version 1.10
Keywords
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type | Subject |
|---|---|---|---|---|---|---|
| Intermolecular Forces and States of Matter - Interactive Lecture Demonstration |
|
|
Ted Clark, Julia Chamberlain | UG-Intro | Demo | Chemistry |
| States of Matter (Inquiry based) Phase Change and Phase diagrams |
|
|
Trish Loeblein | UG-Intro HS |
Lab | Chemistry Physics |
| PhET Sims Aligned to the Chemistry Curriculum |
|
Julia Chamberlain | UG-Intro HS |
Other | Chemistry | |
| Alignment of PhET sims with NGSS |
|
Trish Loeblein | HS | Other | Earth Science Physics Biology Chemistry |
|
| How do PhET simulations fit in my middle school program? |
|
Sarah Borenstein | MS | Other | Physics Biology Chemistry Earth Science |
|
| Mapping of PhET and IBDP Physics | Jaya Ramchandani | HS | Other | Physics | ||
| MS and HS TEK to Sim Alignment | Elyse Zimmer | MS HS |
Other | Biology Chemistry Physics |
||
| The Covalent Bond Between Two Atoms | Roberto Marrero | UG-Intro HS |
HW | |||
| Introduction to the Covalent Bond | Roberto Marrero | HS | HW Lab |
|||
| 원자간 상호작용 SIM 사용지침서 | 이화국(Wha Kuk Lee) | HS UG-Intro |
Demo Lab |
Physics Chemistry |
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|