Reactions & Rates
|
Topics
- Reaction
- Kinematics
- Concentration
- Equilibrium
Description
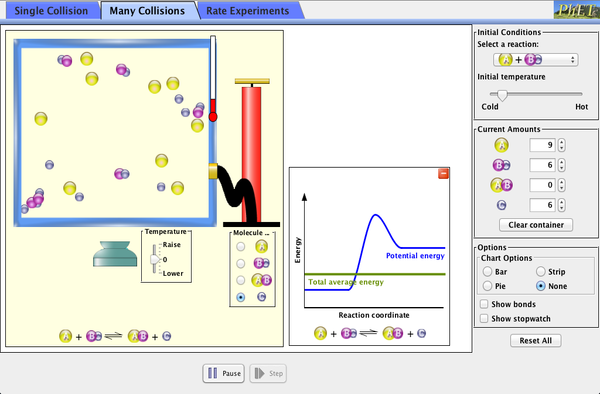
Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, and temperatures. When are reactions reversible? What affects the rate of a reaction?
Sample Learning Goals
- Explain why and how a pinball shooter can be used to help understand ideas about reactions.
- Describe on a microscopic level what contributes to a successful reaction (with illustrations).
- Describe how the reaction coordinate can be used to predict whether a reaction will proceed or slow.
- Use the potential energy diagram to determine: The activation energy for the forward and reverse reactions; The difference in energy between reactants and products; The relative potential energies of the molecules at different positions on a reaction coordinate.
- Draw a potential energy diagram from the energies of reactants and products and activation energy.
- Sketch how the number of reactants and products will change as a reaction proceeds.
- Explain how they know that a system has reached equilibrium from a graph of number of reactants and products versus time.
- Predict how raising or lowering the temperature will affect a system in the equilibrium position.
- Describe the relative sizes of the forward and reverse rates at equilibrium.
- Explain what effects whether the equilibrium position favors the products or the reactants.
- Predict how addition of a reactant or product will affect the forward and reverse reaction rates, and once this new system reaches equilibrium how the reactant and product concentrations will compare to the original system at equilibrium.
- Compare graphs of concentration vs. time to determine which represents the fastest or slowest rate.
Version 1.07
Keywords
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type | Subject |
|---|---|---|---|---|---|---|
| Using PhET in High School Chemistry- all my activities in pdf |
|
|
Trish Loeblein | UG-Intro HS |
Demo Lab HW |
Chemistry |
| Concept Questions for Chemistry using PhET |
|
|
Trish Loeblein | HS UG-Intro MS |
MC | Chemistry |
| Reactions and Rates 4: Equilibrium LeChatlier |
|
|
Trish Loeblein | UG-Intro HS |
Lab HW |
Chemistry |
| Reactions and Rates 4 lessons (Inquiry Based) |
|
|
Trish Loeblein | UG-Intro HS |
CQs Lab Demo HW |
Chemistry |
| Salts and Solubility 5 Activities in pdf |
|
|
Trish Loeblein | HS UG-Intro |
Other | Chemistry Earth Science |
| Reactions and Rates 3: Introduction to Equilibrium (Inquiry Based) |
|
|
Trish Loeblein | UG-Intro HS |
HW Lab CQs |
Chemistry |
| Reactions and Rates 2: Intro to Kinetics (inquiry based) |
|
|
Trish Loeblein | UG-Intro HS |
HW CQs Lab |
Chemistry |
| Reactions and Rates College version for tab 3- kinetics (Inquiry Based) |
|
|
Trish Loeblein | UG-Adv | Lab | Chemistry |
| Reactions and Rates 1 Introduction to reactions (Inquiry Based) |
|
|
Trish Loeblein | UG-Intro HS |
Demo Lab CQs |
Chemistry |
| Exploring Equilibrium 2 (Le Chatelier's Principle) |
|
Amy Jordan | HS | Lab | Chemistry | |
| Exploring Equilibrium |
|
Amy Jordan | HS | Lab | Chemistry | |
| Kinetics Guided Inquiry |
|
Chris Dewberry | UG-Intro | CQs Other HW Lab |
||
| Reaction Rates |
|
Emily Firchau | HS | Lab | Chemistry | |
| PhET Sims Aligned to the Chemistry Curriculum |
|
Julia Chamberlain | HS UG-Intro |
Other | Chemistry | |
| Alignment of PhET sims with NGSS |
|
Trish Loeblein | HS | Other | Biology Physics Earth Science Chemistry |
|
| How do PhET simulations fit in my middle school program? |
|
Sarah Borenstein | MS | Other | Earth Science Physics Biology Chemistry |
|
| Reactions and Rates: Learning Goals from the design team (Inquiry Based) |
|
Trish Loeblein, Linda Koch | HS UG-Adv UG-Intro |
Other | Chemistry | |
| MS and HS TEK to Sim Alignment | Elyse Zimmer | MS HS |
Other | Biology Chemistry Physics |
||
| Understanding Rates of Reactions | Lauren Petersen | MS UG-Intro HS |
Guided Lab HW |
Chemistry | ||
| Reactions and Rates | Jefferson County Middle School Workshop | MS | Lab | Chemistry | ||
| LeChateilier's Principle - Concentration | Robert Strauss | HS | Lab | |||
| 반응과 반응속도 SIM 사용지침서 | 이화국(Wha Kuk Lee) | HS UG-Intro |
Demo Other Lab |
Biology Chemistry |
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|