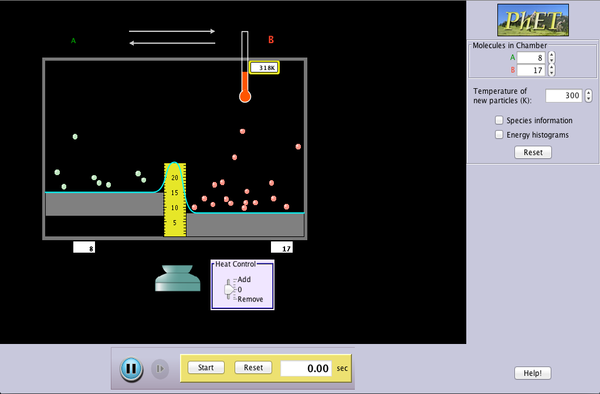
Reversible Reactions
|
Topics
- Thermodynamics
- Temperature
- Heat
- Gas
- Reaction
- Thermal Energy
Description
Watch a reaction proceed over time. How does total energy affect a reaction rate? Vary temperature, barrier height, and potential energies. Record concentrations and time in order to extract rate coefficients. Do temperature dependent studies to extract Arrhenius parameters. This simulation is best used with teacher guidance because it presents an analogy of chemical reactions.
Sample Learning Goals
- Describe on a microscopic level, with illustrations, how reactions occur.
- Describe how the motion of reactant molecules (speed and direction) contributes to a reaction happening.
- Predict how changes in temperature, or use of a catalyst will affect the rate of a reaction.
- On the potential energy curve, identify the activation energy for forward and reverse reactions and the energy change between reactants and products.
- Sketch how the concentrations of reactants and products change as a reaction proceeds.
- From a graph of concentration as a function of time, students should be able to identify when a system has reached equilibrium.
- Calculate a rate coefficient from concentration and time data.
- Determine how a rate coefficient changes with temperature.
- Compare graphs of concentration versus time to determine which represents the fastest or slowest rate.
Version 3.15
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type | Subject |
|---|---|---|---|---|---|---|
| Entropy, Microstates, and Probability - Interactive Lecture Demonstration Guide |
|
|
Ted Clark, Julia Chamberlain | UG-Intro | Demo | Chemistry |
| Salts and Solubility 3: Solution Equilibrium and Ksp (Inquiry Based) |
|
|
Trish Loeblein | HS UG-Intro |
CQs Lab |
Chemistry |
| Inquiry Equilibrium activity |
|
Joshua Manner | HS | Guided Lab |
Chemistry | |
| Equilibrium Inquiry and Experiment Activity |
|
Ryan White | HS | Lab | Chemistry | |
| PhET Sims Aligned to the Chemistry Curriculum |
|
Julia Chamberlain | UG-Intro HS |
Other | Chemistry | |
| Alignment of PhET sims with NGSS |
|
Trish Loeblein | HS | Other | Chemistry Biology Physics Earth Science |
|
| How do PhET simulations fit in my middle school program? |
|
Sarah Borenstein | MS | Other | Earth Science Biology Physics Chemistry |
|
| MS and HS TEK to Sim Alignment | Elyse Zimmer | MS HS |
Other | Physics Chemistry Biology |
||
| Le Chatelier's Principle Demos | Laura Conrad | HS | CQs Lab Demo |
Chemistry | ||
| Basic Thermodynamics Inquiry | Dan Kohler | HS | CQs | Chemistry | ||
| 가역반응 SIM 사용지침서 | Wha Kuk Lee | HS UG-Intro |
Lab Other |
Chemistry |
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|